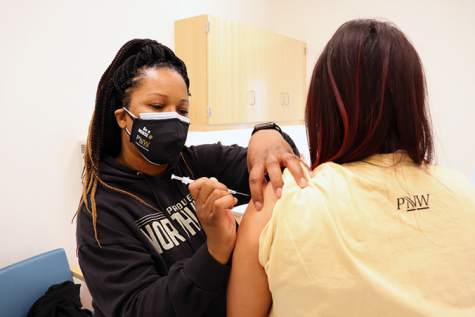
Vaccines may be ready for public distribution soon, but students voice concern about speedy roll-out
A coronavirus vaccine could be widely available by April but some students say they are concerned about the product’s safety and speedy development.
“I’m a little bit hesitant. We don’t know much about it,” said senior communication major Alicia Napules. Right now, three vaccines have been announced from medicine developers Pfizer, Moderna and AstraZeneca. All three companies recently announced that its COVID-19 vaccine may be 90% effective in preventing infection.
“It’s scary. I have personally lost people to the coronavirus,” she continued, adding that she and her family are eager for a return to normalcy after months of living in what she calls a “bubble.”
Still, the mother of three worries about the speed at which the vaccine has been developed and the potential side effects that may arise as a result.
“I would like to see it play out a little longer,” she said. “I’d like to see what statistics and actual evidence has been provided and then we’ll make an educated decision together as a family.”
Political Science major Martha Gallegos admitted that she too is skeptical of receiving the Pfizer vaccine, which is purported to exceed the Food and Drug Administration’s COVID-19 vaccine effectiveness threshold of 50%.
“What I’ve heard is that sometimes these vaccines that are created so quickly sometimes tend to hurt people more in the long run,” she said, adding that she would need to see more information to make a better informed decision.
Gallegos isn’t the only one who feels this way.
A September survey found 49% of U.S. adults said they definitely or probably would not get a COVID-19 vaccination and just 21% said they definitely would. The survey by Pew Research Center found that 72% of those who said they would not get the vaccine cited a need for more information as the major reason behind their decision.
Lindsay Gielda, an assistant professor in the Department of Biological Sciences, believes education is key to making the public more aware of the value of vaccines in preventing the spread of illness.
“If you look over the course of human history, there’s been a number of advancements in society that have allowed us to live longer and have improved our quality of healthcare,” she said. “Vaccines have been one of, if not the, most influential components.”
Gielda said she understands the concerns over the expedited production of Pfizer’s vaccine, but stressed that the development process is working as it should.
“Unfortunately, it is a complex vaccine, which is going to confuse a lot of people, but if they understand and know the biology behind it they’ll see why it’s able to move much faster,” she said.
Pfizer’s announcement of a 90% effective vaccine comes as rates of COVID-19 cases continue to rise in the United States. Nearly 50,000 new cases of infection have been reported in Indiana since Nov., 1, according to the Centers for Disease Control and Prevention. To date, the Indiana Department of Public Health reports more than 240,000 Hoosiers have been infected and over 4,600 have died from COVID-19.
According to a statement by Pfizer, the vaccine will require two doses and protection from the coronavirus is achieved 28 days after the initial vaccination. Phase three trials of the vaccine are ongoing and Pfizer says that no serious safety concerns have been observed. If all goes well, Pfizer expects that 1.3 billion doses of the vaccine will be available in 2021.
Gielda said the news could signal the end of what has been a difficult and deadly year for many, but only if everyone is on board.
“We need to let the medical field be the forefront and the communicators in this process because there is a lot of mistrust right now, sadly, and that’s not a good thing,” she said. “If this vaccine really is as effective as they say, then this would be a way to end the pandemic.”